10 Essential [Mandated] Health Benefits (EBH)
under Health Care Reform, PPACA
ACA Obamacare Essential (Mandatory) Benefits
and #CA California Essential Health Benefits
- CHCF Comparison of CA and Federal Essential Health Benefits
- (A) Ambulatory patient services.
- (B) Emergency services.
- Emergency response ambulance or ambulance transport services
- (C) Hospitalization.
- (D) Maternity [but not infertility - CA Law? ] and newborn care.
- §146.130 Standards relating to benefits for mothers and newborns.
- Maternity: Inpatient hospital and ambulatory
- Prescription drug coverage for contraceptives
- Maternity hospital stay
- Sterilization operations and procedures View the Affordable health ca.com page Assembly Bill 1453 (Monning) and Senate Bill 951 (Hernandez). View the California Health and Safety Code, section 1367.005
- Abortion G-d forbid
- (E) Mental health and substance use disorder services, including behavioral health treatment.
- California Mental health parity
- Autism care (FAQ) including behavioral health treatment
- autism learning partners.com/medi-cal
- insurance.ca.gov/autism
- Sample Policy - use ctrl f to search for autism
- Medi Cal FAQ's
- Autism care (FAQ) including behavioral health treatment
- Biden administration finalizes rules to ensure insurers pay for mental health care
- California Mental health parity
- (F) Prescriptions Drugs – Rx – Including under 65 ACA CFR 156.122
- (G) Rehabilitative and habilitative [learn or improve skills for daily living] services and devices.
- (H) Laboratory services.
- (I) Preventive and wellness services and chronic disease management.
- Diabetes education, management and treatment
- (J) Pediatric services, including Dental - oral and vision care. Blue Shield Individual Flyer Essential Health Benefits 5.2013 Group Essential Health Benefits (EBH) 42 USC 18022 SB 951
- (H?) Cancer and other life threatening disease - clinical trials
- AIDS vaccine
- HIV testing
- Organ transplants for HIV
- Alpha feto protein testing
- Prosthetics for laryngectomy
- Reconstructive surgery
- Mastectomies and lymph node dissections
- Cervical cancer treatment
- Osteoporosis treatment
- Surgical procedures for jaw bones
- Anesthesia for dental
- Conditions attributable to diethylstilbestrol
- Hospice (end of life) care
- Pain management medication for terminally ill
- Phenylketonuria treatment
- Health Care.Gov
- CMS.gov very detailed
-
White House.Gov Affordable Health Care Act YouTube Channel
- Here's the Feb. 20, 2013 final.rule establishing the essential health benefits (EHBs) for 10 categories of care, including basic services such as hospitalization and emergency care, as well as mental health and maternity care. In addition, the plans must cover a minimum of 60 percent of the actuarial value of covered medical services.
-
No more Annual & Lifetime Limits under Health Care Reform Aetna's Explanation
- § 1300.67.005. Essential Health Benefits Westlaw Barclays California Code of Regulations
- CHCF Comparison of CA and Federal Essential Health Benefits
- CA Department of Insurance
- Covered CA
- Medi Cal Listing
- California Benchmarks Kaiser Small Group HMO 30 ♦ CA SB 43 effective 1.1.2016 makes this the benchmark for individual plans too.
- CHCF Comparison of CA vs Federal Rules as of 2014
- Frequently Asked Questions on Essential Health Benefits Bulletin from the Department of Health and Human Services (PDF)
- Essential Health Benefits Bulletin from the Department of Health and Human Services, Dec. 16, 2011 (PDF)
-
No more Annual & Lifetime Limits under Health Care Reform Aetna's Explanation
- Short Term Policies that don't have essential benefits have been banned in CA for a long time. Now the Feds are doing it. Kff.org
- Preventive services for children 16 and younger
Mental health parity
Autism care including behavioral health treatment
Prescription drug coverage for contraceptives
Maternity hospital stay
Maternity: Inpatient hospital and ambulatory
AIDS vaccine
HIV testing
Organ transplants for HIV
Diabetes education, management and treatment
Alpha feto protein testing
Prosthetics for laryngectomy
Reconstructive surgery
Mastectomies and lymph node dissections
Mammography
Cervical cancer treatment
Cancer screening tests
Cancer clinical trials
Prostate cancer treatment
Osteoporosis treatment
Surgical procedures for jaw bones
Anesthesia for dental
Conditions attributable to diethylstilbestrol
Hospice (end of life) care
Pain management medication for terminally ill
Emergency response ambulance or ambulance transport services
Medicare Coverage # 11021
Phenylketonuria treatment
Sterilization operations and procedures View the Affordable health ca.com page Assembly Bill 1453 (Monning) and Senate Bill 951 (Hernandez). View the California Health and Safety Code, section 1367.005 - Our main webpage on California & Federal Essential Health Benefits
- jump to section on:
Specimen Individual Policy #EOC with Definitions
Employer Group Sample Policy
It's often so much easier and simpler to just read your Evidence of Coverage EOC-policy, then look all over for the codes, laws, regulations etc! Plus, EOC's are mandated to be written in PLAIN ENGLISH!
- Find your own Individual EOC Evidence of Coverage
- It' important to use YOUR EOC not just stuff in general!
- Obligation to READ your EOC
- Plain Meaning Rule - Plain Writing Act
- Our Webpage on Evidence of Coverage
- OOP Out of Pocket Maximum - Many definitions are explained there.
VIDEO Steve Explains how to read EOC
All our plans are Guaranteed Issue with No Pre X Clause
Quote & Subsidy #Calculation
There is No charge for our complementary services
Watch our 10 minute VIDEO
that explains everything about getting a quote
- Our Quote Engine Takes all the complexity out of using pencil and paper to figure out the premiums per the Obamacare/ACA rules under CFR §1.36B-3 *
- Get more detail on the Individual & Family Carriers available in CA
Lab Fees
What and how much is covered for #Lab – Laboratory Fees – as an essential benefit under Health Care Reform?
Is the $10 limit for most X-rays and laboratory test limited only to this Blue Shield Platinum plan?
We grant this page isn’t written in plain English, but is a cut & paste from an email… Ask your questions below and the formatting will look better as web design tools have improved.
***I believe the co-pay would depend on what Metal Level, one has purchased.
Are the words “most” and “encounter” defined anywhere?
***Proactive Office Encounter is a Kaiser Term – Here’s an explanation… but I don’t find a simple definition.
I see that essential benefits include laboratory tests for PSA, cholesterol and diabetes are no charge items as they are deemed preventative care.
***Yes, but see the limitation for conditions that are already diagnosed!
Our current Blue Shield of CA PPO small business plan took effect on July 1, 2011. As I have previously explained, when I last had routine lab tests, which was in October, 2012, the cost was over $1,000 from an in-network provider. So, since the Affordable Care Act has taken more full effect, is there any chance those lab tests which are now deemed preventative care and thus no charge,
***I’m glad you’ve asked this question… I don’t think ANYONE knows of the limitation for what’s already been diagnosed.
will be no charge under our current plan, or will we have to wait until a new plan is in effect?
Lab Fees are an essential benefit.
Preventive Care
Preventive care is given during an Office Visit or as an outpatient. Screenings and other services are covered for adults and children with no current symptoms or history of a health problem. Members who have current symptoms or a diagnosed health problem will get benefits under the “Diagnostic Services” (Page 74) benefit, not this benefit. – Page 92
Office Visits
An Office Visit is when You go to a Physician’s office and have one or more of ONLY the following three services provided:
-
History-Gathering of information on an illness or injury.
-
Examination
-
Physician’s medical decision regarding the diagnosis and treatment plan.
Office Visit will not include any other services while at the office of a Physician (e.g., any surgery, Infusion Therapy, diagnostic X-ray, laboratory, pathology and radiology) or any services performed other than the three services specifically listed above.
Covered Services include:
Office Visits with Primary care Physicians and Providers (PCP) and Specialty Care Physicians and Providers
Home Visits for medical care to examine, diagnose, and treat an illness or injury.
Note: Physician visits in the home are different than the “Home Care Services” benefit described earlier in this Agreement (Page 88)
Sample Evidence of Coverage EOC Platinum Level
USC United States Code on Essential Benefits
149 page final.rule_.essential.health.benefits
benchmark.Kaiser Small Group HMO
One might also consider these options for fees that don’t get paid
health-savings-accounts-hsa/
Section 105 medical_expense_reimbursement_plans_section_105.
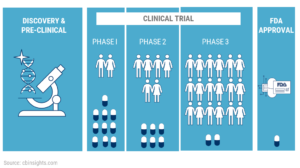
Clinical Trial
#Clinical Trials are Covered under Health Care Reform
- Health Care Reform/ACA/Obamacare requires health plans to pay the routine care costs of patients who participate in clinical trials for the prevention, detection and treatment of cancer and other life-threatening conditions. Read the entire article ⇒ Kaiser Health News – Simple Explanation 8.24.2010Aetna.com explanation of how clinical trials are an essential benefit under Health Care Reform
- Specimen Platinum Policy – Clinical Studies Page 71
- Experimental Exclusion Page 109 163
- Independent Medical Review Page 153
- Learn more About Clinical Studies including relevant History, Policies, and Laws.
- United Health Care
- CIGNA Fact Sheet
- Clinical Trials.Gov ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world.
- The problem wasn’t the costs of the clinical trial itself:
- The cancer center would pay to administer the drug and analyze the results. But if Crusoe participated in the trial, his health plan would stop covering all the other doctor visits, hospital stays, tests and treatment related to treating his cancer. Routine patient care refers to the range of medical services people with a particular diagnosis might need. It includes treatment for side effects and other medical issues that might arise as a result of the trial. Although Medicare and many private health plans already cover such costs, some plans decline to do so on the grounds that clinical trials are experimental, say experts. More than half of states require coverage of routine costs in a clinical trial, but state requirements vary. The new law sets a minimum standard. section 2709(a) *
Definitions
Life Threatening Conditions
- ‘life-threatening condition’ means any disease or condition from which the likelihood of death is probable unless the course of the disease or condition is interrupted. (of an illness, situation, etc) that makes it possible that the person affected will die ‘‘§2709 (e) * Collins Dictionary.com
- Links & References
- Federal Register Ryan White Regulations Section 2695 42 USC 300ff-131
- 42 USC 300gg-8 Coverage for individuals participating in approved clinical trials
CA Insurance Codes for clinical studies related to cancer
chbrp.org Health & Safety Code 1370.6
Insurance Code 10145.4
- (a) For an insured diagnosed with cancer and accepted into a phase I, phase II, phase III, or phase IV clinical trial for cancer, every policy of disability insurance that provides hospital, medical, or surgical coverage in this state shall provide coverage for all routine patient care costs related to the clinical trial if the insured’s treating physician, who is providing covered health care services to the insured under the insured’s health benefit plan contract, recommends participation in the clinical trial after determining that participation in the clinical trial has a meaningful potential to benefit the insured. For purposes of this section, a clinical trial’s endpoints shall not be defined exclusively to test toxicity, but shall have a therapeutic intent.
- (b) (1) “Routine patient care costs” means the costs associated with the provision of health care services, including drugs, items, devices, and services that would otherwise be covered under the plan or contract if those drugs, items, devices, and services were not provided in connection with an approved clinical trial program, including the following:
- (A) Health care services typically provided absent a clinical trial. [Essential Benefits]
- (B) Health care services required solely for the provision of the investigational drug, item, device, or service.
- (C) Health care services required for the clinically appropriate monitoring of the investigational item or service.
- (D) Health care services provided for the prevention of complications arising from the provision of the investigational drug, item, device, or service.
- (E) Health care services needed for the reasonable and necessary care arising from the provision of the investigational drug, item, device, or service, including the diagnosis or treatment of the complications.
- [Medical Necessity * Clinical Guidelines UM Utilization Management]
- (2) For purposes of this section, “routine patient care costs” does not include the costs associated with the provision of any of the following:
- (A) Drugs or devices that have not been approved by the federal Food and Drug Administration and that are associated with the clinical trial.
- (B) Services other than health care services, such as travel, housing, companion expenses, and other nonclinical expenses, that an insured may require as a result of the treatment being provided for purposes of the clinical trial.
- (C) Any item or service that is provided solely to satisfy data collection and analysis needs and that is not used in the clinical management of the patient.
- (D) Health care services which, except for the fact that they are not being provided in a clinical trial, are otherwise specifically excluded from coverage under the insured’s health plan.
- (E) Health care services customarily provided by the research sponsors free of charge for any enrollee in the trial.
- (c) The treatment shall be provided in a clinical trial that either (1) involves a drug that is exempt under federal regulations from a new drug application or (2) that is approved by one of the following:
- (A) One of the National Institutes of Health.
- (B) The federal Food and Drug Administration, in the form of an investigational new drug application.
- (C) The United States Department of Defense.
- (D) The United States Veterans’ Administration.
- (d) In the case of health care services provided by a contracting provider, the payment rate shall be at the agreed-upon rate. In the case of a noncontracting provider, the payment shall be at the negotiated rate the insurer would otherwise pay to a contracting provider for the same services, less applicable copayments and deductibles. Nothing in this section shall be construed to prohibit a disability insurer from restricting coverage for clinical trials to hospitals and physicians in California unless the protocol for the clinical trial is not provided for at a California hospital or by a California physician.
- (e) The provision of services when required by this section shall not, in itself, give rise to liability on the part of the insurer.
- (f) This section shall not apply to vision-only, dental-only, accident-only, specified disease, hospital indemnity, Medicare supplement, CHAMPUS supplement, long-term care, or disability income insurance, except that for specified disease and hospital indemnity insurance, coverage for benefits under this section shall apply, but only to the extent that the benefits are covered under the general terms and conditions that apply to all other benefits under the policy. Nothing in this section shall be construed as imposing a new benefit mandate on specified disease or hospital indemnity insurance.
- (g) Nothing in this section shall be construed to prohibit, limit, or modify an insured’s rights to the independent review process available under Section 10145.3 or to the Independent Medical Review System available under Article 3.5 (commencing with Section 10169).
- (h) Nothing in this section shall be construed to otherwise limit or modify any existing requirements under the provisions of this chapter or to prevent application of deductible or copayment provisions contained in the policy.
- (i) Copayments and deductibles applied to services delivered in a clinical trial shall be the same as those applied to the same services if not delivered in a clinical trial.
- (Amended by Stats. 2002, Ch. 664, Sec. 156. Effective January 1, 2003.)
Coverage for Individuals Participating in Approved Clinical Trials
- In general, PHS Act section 2709(a),6 as added by the Affordable Care Act, states that if a group health plan or health insurance issuer in the group and individual health insurance market provides coverage to a qualified individual (as defined under PHS Act section 2709(b)), then such plan or issuer:
- (1) may not deny the qualified individual participation in an approved clinical trial with respect to the treatment of cancer or another life-threatening disease or condition;
- (2) may not deny (or limit or impose additional conditions on) the coverage of routine patient costs for items and services furnished in connection with participation in the trial; and
- (3) may not discriminate against the individual on the basis of the individual’s participation in the trial.
- A qualified individual under PHS Act section 2709(b) is generally a participant or beneficiary who is eligible to participate in an approved clinical trial according to the trial protocol with respect to the treatment of cancer or another life-threatening disease or condition; and either: (1) the referring health care professional is a participating provider and has concluded that the individual’s participation in such trial would be appropriate; or (2) the participant or beneficiary provides medical and scientific information establishing that the individual’s participation in such trial would be appropriate.
- Q3: Will the Departments be issuing regulations addressing PHS Act section 2709 prior to its effective date?
- No. The statutory language of PHS Act section 2709 is self-implementing and the Departments do not expect to issue regulations in the near future. PHS Act section 2709 is applicable to non-grandfathered group health plans and health insurance issuers offering group or individual health insurance coverage for plan years (in the individual market, policy years) beginning on or after January 1, 2014.
- Until any further guidance is issued, group health plans and health insurance issuers are expected to implement the requirements of PHS Act section 2709 using a good faith, reasonable interpretation of the law. The Departments will work together with employers, plans, issuers, states, providers, and other stakeholders to help them come into compliance with the law and will work with families and individuals to help them understand the coverage for clinical trials provision and benefit from it as intended.
- For questions about the coverage for clinical trials provision, including complaints regarding compliance with the statutory provision by health insurance issuers, contact your state department of insurance (contact information is available by visiting healthcare.gov or the Centers for Medicare & Medicaid Services, Center for Consumer Information and Insurance Oversight at 1-888-393-2789. For employment-based group health plan coverage, you also may contact the Department of Labor at www.askebsa.dol.gov or 1-866-444-3272.
- PHS Act section 2709 also is incorporated into section 715(a)(1) of ERISA and section 9815(a)(1) of the Code. Accordingly, the Departments have concurrent jurisdiction over the implementation of PHS Act section 2709. Copied from CMS.gov
Technical
Guidence – Bulletins Rules CMS.Gov
View the details and background of Essential Benefits on SteveShorr.com Future updates will be on this site.
Secretary of Health Kathleen Sebelius issued a set of defined “essential health benefits”[22] that all new insurance plans have to include. Insurers will be prohibited from imposing annual or lifetime coverage caps on these essential benefits.[191][195] These cover: “ambulatory patient services; emergency services; hospitalization; maternity and newborn care; mental health and substance use disorder services, including behavioral health treatment; prescription drugs; rehabilitative and habilitative services and devices; laboratory services; preventive and wellness services and chronic disease management; and pediatric services, including oral and vision care.”[196][197] In determining what would qualify as an essential benefit, the law required that the scope of standard benefits should equal that of a “typical employer plan”.[196] States have some discretion in determining what should be considered the benchmark plan within the requirements of the law, and may include services beyond those set out by the Secretary.[198]
Among the essential health benefits, preventive care, childhood immunizations and adult vaccinations, and medical screenings will be covered by an insurance plan’s premiums, and have co-payments, co-insurance, and deductibles eliminated.[199][200] Specific examples of such services covered include: mammograms and colonoscopies, wellness visits, gestational diabetes screening, HPV testing, STI counseling, HIV screening and counseling, FDA-approved contraceptive methods, breastfeeding support and supplies, and domestic violence screening and counseling.[201] Wikipedial
Subpart B—Essential Health Benefits Package
Proposed Changes CMS 9930 P 10.27.2017
§156.100 State selection of benchmark.
§156.105 Determination of EHB for multi-state plans.
§156.110 EHB-benchmark plan standards.
§156.115 Provision of EHB.
§156.120 Collection of data to define essential health benefits.
§156.122 Prescription drug benefits.
§156.125 Prohibition on discrimination.
§156.130 Cost-sharing requirements.
§156.135 AV calculation for determining level of coverage.
§156.140 Levels of coverage.
§156.145 Determination of minimum value.
§156.150 Application to stand-alone dental plans inside the Exchange.
§156.155 Enrollment in catastrophic plans.
Health Insurance unfortunately is very complicated
President Trump February 27, 2017
- Thus, if we haven't simplified and explained in PLAIN ENGLISH what you are looking for:




Does ACA/Obamacare cover hearing aids?
We will answer that in our hearing aid section
Where can I get a detailed coverage guide for Kaiser Individual Plans?
Check our Kaiser Individual Webpage